This is the second issue of our monthly Life Science Patent Highlights. Whenever possible we are now linking to ChemSpider for chemical structures and key data of investigational compounds. You can perform a lot of deeper chemistry-based searches on this free platform.
PLEASE CITE AS: Mucke, HAM. Patent Highlights for January 2011. H.M. Pharma Consultancy. URL:http://hmpharmacon.blogspot.com/2011/02/patent-highlights-for-january-2011.html. WebCite archive link: http://www.webcitation.org/5wAQpr2tN. Contact us at office@hmpharmacon.com.
Boron-based Insulin Adjusts Activity To Glucose Levels
Originating from the leader in insulin medications, an application that is titled “insulin derivatives” (WO 2011/000823, Novo Nordisk [DK]) would seem boring: OK, what else is new? A lot actually. These modified insulins sense glucose levels, and become fully active only when glucose levels exceed 10 mM (i.e., under conditions of hyperglycemia) while they remain totally inactive at 3 mM and below. (In healthy persons, the blood glucose concentration is about 5 mM, rising to about 7 mM postprandially.) The company has started claiming insulin derivatives with such properties a decade ago; see WO 2001/092334 and WO 2003/048195. Why now boron? Due to its strong interaction with diol moieties, the boronic acid group is frequently employed in glucose sensors (J Fluoresc. 2004;14(5):481-9; here); and – in a twist that is perhaps more remote – physiologic amounts of boron may reduce insulin requirements (J. Nutr. 2003; 133:3577-83 – PDF).
Chromium for Glucose Transport Into The Brain
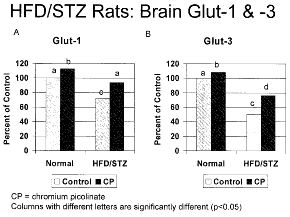
In WO 2011/002939 Nutrition 21, Inc. [US], Jan. 6, 2011) shows that bioavailable complex salts of trivalent chromium, such as the picolinates and histidinates, can enhance the expression as well as the function of GLUT1 (the insulin-independent primary glucose transporter in the blood-brain barrier) and GLUT3 (the neuronal glucose transporter). Streptozotocin-treated rats fed a high fat diet (the HFD/STZ model for type 2 diabetes) significantly restored their reduced increased brain and serum chromium levels, increased brain GLUT-1 and GLUT-3 levels towards normalcy, and decreased body weight and blood glucose levels, compared to the high fat diet alone. A Turkish group has pre-published results to the same effect in December 2010 (see here). This could have implications for nutrition in diabetes and dementia patients. Nutrition 21’s flagship nutritional supplement product, Chromax®, is chromium picolinate.
A Bioactive Glass For Bone Infections
Chronic osteomyelitis, a typical late complication of fractures and bone implant surgery that is most often caused by S. aureus, is probably one of the most difficult-to-treat infections. Biodegradable inserts that locally release antibiotics are commonly used after debridement. WO 2011/001028 (BonAlive Biomaterials Oy [FI], Jan. 6, 2011) illustrates an alternative non-pharmacological approach: bioactive glass pastes that release sodium, calcium, phosphate and silicate. These ions can elevate the pH of the local medium to extremely alkaline values (11-12) and they also perturb the bacterial membrane potential. Moreover, such implants can stimulate bone growth and angiogenesis for long periods. BAG 53SP4, a bioactive glass from Vivoxid Ltd, a Finnish medical technology company in the bone reconstruction and augmentation business, is referenced in this application. Actually, one of Vivoxid’s three main technologies is called BonAlive and the applicant’s managers also come from Vivoxid, which has obviously spun out the business. In 2008, Vivoxid received 510(k) regulatory clearance for BonAlive bioactive glass in USA.
Addiction Vaccines Reloaded – With A Twist
Vaccination to support smoking cessation or to take the “kick” out of drugs of abuse is a decade-old concept. The challenge is to make nicotine, cocaine or amphetamines immunogenic so that neutralizing antibodies can be induced; they need to be conjugated to a hapten and to a carrier (see a review here). While nicotine vaccination approaches met with little luck in clinical development in the 1990s, Nabi Biopharmaceuticals’ NicVAX® commenced Phase III in November 2009, and results are anticipated by the third quarter of 2011. This new document, WO 2011/000889 (William Henry [GB], Jan. 6, 2011), adds an interesting facet by using the transmucosal route. A bacterial toxin carrier is employed which is able to induce a mucosal immune response by stimulating the vaccinee’s T cells, which in turn help the B cells initiate and maintain sustained antibody production to portions of the entire conjugate, including the hapten portion. Running a comparison of the individual inventor’s name against the known executives and advisory board members of companies who are active in this field identified him as a member of the development team at Celtic Pharma, a Bermuda company which acquired Xenova Group plc and brought their nicotine vaccine, TA-NIC, into a large Phase II clinical trial in 2007 (clinicaltrials.gov code: NCT00633321). The study was completed in Februry 2009 but no results were reported by the company. TA-NIC uses a recombinant cholera toxin as a hapten-carrier, which confirms this association. Celtic also has a cocaine vaccine, TA-CD, in development and indeed the scope of this patent application spans several drugs of abuse beyond nicotine.
An Agent For Restoring Lost Memory
In one episode of the 2009 mystery TV series FlashForward, the hero is advised to undergo treatment with a calcineurin antagonist so that he might remember the entire 137 seconds of his personal vision of the future. The producers must have had good scientific advice: calcineurin antigonists are actually under investigation for this purpose, e.g. in patients with traumatic brain injury (see here). A more common approach to enhance long-term memory potentiation and cognitive recovery is represented by AMPA receptor agonists, and this is what WO 2011/006653 (AC Immune [CH] & IPAC [RU], Jan. 20, 2011) is demonstrating with a series of diazabicyclononanes that had been identified as having this type of activity in the 2006 Russian patent RU 2333211. In a chicken model of passive avoidance training and subsequent memorydisruption by the protein synthesis inhibitor anisomycine, 3,7-bis(1,3-benzodioxol-5-ylcarbonyl)-1,5-dimethyl-3,7-diazabicyclo[3.3.1]nonane-9-one prevented amnesia. The patent claims extend to other states of cognitive disturbance such as Alzheimer’s disease.
Vaginal Delivery of Oxybutynin
Vaginal drug delivery has been traditionally regarded in the context of local medical conditions, especially infections and for the prevention of sexually transmitted diseases, with the later addition of spermicidal compounds to serve as local contraceptives. That significant systemic absorption of drugs can occur through the vagina is a relatively recent insight. WO 2011/005709 (Femmepharma [US], Jan. 13, 2011) offers vaginal gels that are applied small amounts (< 1 ml) and deliver a drug to treat bladder dysfunction. Preferably this is oxybutynin, an antimuscarinic which is one of the most widely prescribed oral medications for the treatment of stress and urge incontinence. In a clinical study, 15 women were dosed with 0.2 ml Oxybutynin HCl intravaginal gel (4 mg/day) for 21 days. Subjects recorded in a diary for 14 days of screening prior to drug treatment and then during the 21 days of treatment the number of micturitions per day and the number of incontinence events. The number of events decreased to 11.6 +/- 3.2 micturitions/day and 0.4 +/- 0.6 accidents per day during the third week on treatment, compared to 15.4 +/- 7.40 micturitions/day and 1.2 +/- 1.4 accidents/day, respectively at baseline (P <0.05). Importantly, the typical anti-muscarinic side-effects such as dry mouth were absent. Apparently that refers to a Phase II study (NCT00749632) that was completed in 2008 but had no peer-reviewed results published. Work on pervaginal oxybutynin first appeared in the literature in 1996 (see here).
PPARgamma Ligands For Aortic Aneurysm
GlaxoSmithKlines thiazolidinedione insulin sensitizer Avandia (rosiglitazone, a pure PPARgamma ligand) had a good start but is in deep troubles now: the EMEA recommended its European market withdrawal in September 2010, and the drug has been hanging on its fingernails in the U.S. for the past three years. GSK announced in January 2011 that it would set aside no less than $3.5 bn. for charges linked to claims about rosiglitazone being linked to myocardial ischemia, heart failure and stroke. However, PPARgamma is not only expressed on fat cells but also in the vascular endothelium. As early as 2002 this has led to claims that downregulation of this anti-inflammatory receptor in the vessel walls is involved in the formation of aortic aneurysms driven by angiotensin-II (see here). WO 2011/007565 (Hamamatsu University School of Medicine [JP]; Jan. 20, 2011) suggests that there might be a twist to that — but with antagonists: “It was discovered that a circulatory failure within the vascular wall cause the fibroblasts constituting abdominal aortic aneurysmal adventitia to express PPARgamma and differentiate into adipocyte-like cells, which induces the abnormal accumulation of triglycerides (…) and weakens the vascular wall. The PPAR inhibitor comprehensively corrects these factors.”The research compounds GW-9662, G-3335, HX-531 and T-0070907 are named as potentially useful PPARgamma blockers. On the other hand, a paper titled “Rosiglitazone reduces the development and rupture of experimental aortic aneurysms” was published here, three weeks before the Japanese parent application was filed in July 2009; this would point exactly in the opposite direction because rosiglitazone is usually conceived as an agonist. But things might not be so clear-cut with PPARgamma.
Bacteria As Oral Gene Therapy Delivery Agents To Tumors
Anaerobic bacteria such as Bifidobacterium and Clostridium have been demonstrated to localise to and replicate in hypoxic tumour tissue when intravenously administered in rodent models. The innovation in WO 2011/007007 (University College Cork [IE]; Jan. 20, 2011) lies in the harnessing of bacterial translocation (the trafficking of viable bacteria from the gastrointestinal tract to extraintestinal sites, a phenomenon that is well studied in the context of sepsis) to achieve this through oral administration. Using the non-pathogenic, food-grade Bifidobacterium breve strain UCC2003 the inventors could show in a mouse model of cancer (pulmonary metastases from B16 melanoma cells) that free bacteria were transmitted from the gut through serum to tumors, and were able to express marker proteins there. The delivered agent encoded by the bacterial vector could be an anti-angiogenic or pro-apoptitic protein (e.g. endostatin, angiostatin, IL12, IL23, IL32), a cytokine, or a recombaint antibody. See here for a September 2010 paper from the David Geffen School of Medicine at UCLA, authored by one of the inventors named on the patent application.
A Heart Failure Drug Repurposed For Osteoporosis
Korean was adopted as a PCT publication language in 2007 and though we cannot read more than the abstract in WO 2011/008052 (Korea Research Institutes of Bioscience and Chemical Technology [KR]; Jan. 20, 2011) this is enough to see that we have an interesting example of potential drug repurposing here. The subject compound, colforsin daropate (6-(3-dimethylaminopropionyl)forskolin; NKH477) is an adenylate cyclase activator that was developed and launched in Japan (as AdehlTM) for the treatment of acute heart failure by Nippon Kayaku Co., Ltd. Now we hear that it “suppresses the differentiation of osteoclasts and bone resorption caused thereby and promotes the differentiation of osteoblasts and the activity thereof….” The drug’s parent compound, forskolin (confusingly sometimes also referred to as colforsin), is a labdane diterpene and the main active ingredient in the ayurvedic herb Coleus forskohlii. It is a frequent subject in heath and nutritional supplement blogs. This should not deter pharmacological interest from the possibility that colforsin daropate might be an interesting lead for osteoporosis, a potential for which we have seen no indication in the peer review literature. For a recent paper on the two compounds see here.
A Disease-Modifying Approach to Schizophrenia
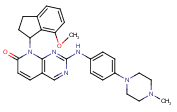
Group I p21-activated kinases (PAK) have crucial roles in cell migration and cytoskeleton dynamics. Their role in axonal growth and guidance has been acknowledged (see here and here). On the other hand, the role of misguided dendritic spine development and cortical synaptic instability in schizophrenia is well appreciated (see here). WO 2011/009097 (Afraxis Inc. [US]; Jan. 20, 2011) make the IP claims concerning this aspect of the company’s focus, which extends to autism, Fragile X syndrome, and Alzheimer’s disease. The document presents several series of substituted pyrido[2,3-d]pyrimidin- 7(8H)-ones as PAK inhibitors, and gives a detailed outline for a 6-week, randomized, double blind, parallel groups study (active comparator: haloperidol) of an oral PAK1/PAK3 inhibitor in 30 patients. No matching entry can yet be found on clinicaltrials.gov. One specific example is 8-(7-methoxy-2,3-dihydro-1H-inden-1-yl)-2-(4-(4- methylpiperazin-1-yl)phenylamino)pyrido [2,3-d] pyrimidin-7(8H)-one (see figure).
Biomarkers For Sepsis, Asthma, And Alzheimer’s
Wheezing children can have several different respiratory conditions; only clinical observations and lung function tests can show conclusively if these are symptoms of actual asthma. While it has been known for some time that the breath of asthmatics contains biomarkers, from simple ethane and pentane (see here) to exhaled proteins (see here), these are not conclusive. WO 2011/003922 (Maastricht University [NL]; Jan. 13, 2011) presents new volatile single-compound markers, all easily assessed by GC/MS, which are supposed to provide diagnostic differentiation. Those with the best discrimination potential are a branched hydrocarbon (C13H28), carbon disulfide; and 1-penten-2-one.
WO 2011/006911 (Bruker Daltonik [DE]; Jan. 20, 2011) also employs mass spectroscopy, but its purpose is the characterization of bacterial pathogens from blood based on the MALDI–TOF profiles of their key proteins. Following brief culture, relatively strong tensides (normally considered problematic for subsequent MALDI because they inhibit ionization) are used to destroy the blood cells. Pathogens can be obtained in their pure form by centrifuging or filtration, and can be identified at least on the species level.
WO 2011/009967 (Universidad Completense de Madrid [ES]; Jan. 27, 2011) demonstrates an application for the key biomarker for Alzheimer’s disease, amyloid Aβ(1-42) which is clever, though limited to patients undergoing cataract surgery: the proteinaceous waste produced by the phacoemulsification procedure contains elevated amounts of this pathological version of amyloid in Alzheimer patients, even before the appearance of cognitve symptoms. The search report dilligently points to a Lancet paper published in 2003 (see here), and to two patent applications (WO 2008/115197 and EP 1913866), to question novelty.
Galantamine Continues To Attract Attention
Sometimes things go full circle… or in something like a spiral. In the mid-1990s Shire plc had been repurposing galantamine for Alzheimer’s disease in an effort that was subsequently completed by Johnson & Johnson’s Janssen Pharmaceutica, and made an unsuccessful attempt in chronic fatigue syndrome. With WO 2011/011766 (Shire [US]; Jan. 27, 2011) the company re-enters the game with prodrugs having side chains of valine, phenylalanine, tyrosine or mono-, di- and tripeptides, or p-amino benzoic acid. The German company Galantos is also working on galantamine prodrugs (see WO 2009/127218); one candidate, Memogain(R), has been in Phase I for some time.
Terbutaline in Diabetes
WO 2011/009818 (Jagotec [CH]; Jan. 27, 2011) claims formulations of terbutaline and other adrenergic beta2 receptor agonists (normally used to treat respiratory diseases) to prevent nocturnal hypoglycemia in insulin-treated diabetics. Before you cry, “Not new!” and point to papers such as J Clin Endocrinol Metab. 2006; 91(6):2087-92 (see here) and Diabetes Care 2008; 31(12):2271-2 (see here), look again: what is actually claimed is a chronotherapeutic fomulation that is supposed to prevent hyperglycemia in the morning. The science of controlled release formulation can provide innovations that are at least as practical as new drugs.