Here are five new drug repurposing patent applications picked and commented for you, (almost) fresh online on PatentScope! – Please attribute to H.M. Pharma Consultancy when citing from this post.
And we are happy to announce two very positive events:
(2) Today we renewed our agreement with the Future Science Publishing Group that secures continued publication of our bimonthly reviews of remarkable PCT patent applications in the Pharmaceutical Patent Analyst throughout 2013. Be sure to take a look at this exciting new journal.
Follow @hmpharmaconon Twitter to receive constant updates on H.M. Pharma Consultancy’s non-confidential activities, and on what we simply find interesting. Among this weeks new Twitter followers we extend special greeting to @SanofiUSand @openscience.
Treating Damaged Mucosa Everywhere: An Obvious Case of Repurposing
Rebamipide, a 2(1H)-quinolinone with multiple mechanisms including matrix metalloproteinase inhibition and prostaglandin induction, was discovered by Otsuka which first launched it in Japan in 1990 as an anti-gastric ulcer agent (Mucosta® tablets). In January 2012, Mucosta® ophthalmic suspension UD2% was launched in Japan for the treatment of dry eye syndrome (see WO/2006/05218 and WO/2008/050896). The tablets (100 mg t.i.d.) also performed well in the treatment of dry mouth in patients with Sjögren’s syndrome (Mod Rheumatol 2009; 19:114-24; PubMed). In WO/2012/128394(Otsuka Pharmaceutical [JP]; Sept. 27, 2012) the developer has taken a quite obvious next step, and claims viscous oral swishing and gargling formulations containing non-aggregated rebamipide nanoparticles for stomatitis caused by salivary deficiencies that occur as side effects of antiproliferative radiotherapy. Especially head and neck cancer patients suffer severely from mucosal damage and dental deterioration.
TC-5619 – A Failed Attempt At Originator-Initiated Repurposing
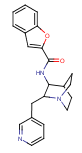 |
| TC-5619 |
It takes a bit of research to find out about the multifaceted history of (2S,3R)-N-(2-((3-pyridinyl)methyl)-1-azabicyclo[2.2.2]oct-3-yl)benzofuran-2-carboxamide, the agent claimed for attention deficit disorder (ADHD) in WO/2012/129262(Targacept [US]; Sept. 27, 2012). The racemic compound, which contains two asymmetrically substituted carbon atoms, was first claimed as a nicotinic receptor (nAChR) ligand in US Patent No. 6,953,855. The two enantiomers with the trans relative stereochemistry differ substantially from one another in their ability to interact with the α7 nAChR subtype. The molecule with the (2S,3R) configuration, TC-5619, has the greater activity (≤1 nM Ki value); it is a highly selective, full agonist with a remarkably low EC50 value for receptor activation, and with a good separation towards the IC50 for residual inhibition. It demonstrated efficacy in animal models of the positive and negative symptoms and cognitive dysfunction of schizophrenia (Biochem Pharmacol 2009; 78(7): 803-12; see here), and in a Phase II clinical trial. Targacept had also clinically investigated TC-5619 for ADHD, but by March 2011 (the time of the first of the present patent application’s three priority dates) it had already been clear that the trial had missed its efficacy endpoint. So why still go ahead with an ADHD patent application? Because a subgroup analysis had shown efficacy signals on the primary outcome variable in patients with inattentive-predominant ADHD. Another 13-site Phase II trial was conducted that focused on this patient subgroup. However, in September 2012 Targacept had to announce complete failure: across all outcome measures, patients in the placebo group consistently improved more than those in the TC-5619 groups. Well, nobody ever claimed that repurposing or use extension de-risks development in terms of efficacy…
D-Propranolol And Desipramine For EGFR-Driven Cancers
A Phase III clinical study sponsored by Sheba Medical Center (NCT00888797) is currently investigating the old beta-blocker propranololin combination with the cyclooxygenase-2 inhibitor etodolac in patients undergoing surgery for colon and rectal cancer. However, the rationale of this study is rooted in perioperative stress reduction, for which the beta-adrenergic antagonism of L-propanolol is solely responsible. The inventors of WO/2012/135970Universidad Catolica de Chile [CL]; Oct. 11, 2012) have now shown that both L- and D-isomers inhibit phosphatidic acid phosphohydrolase (PAP) which is part of TLR-mediated inflammatory signaling (Adv Enzyme Regul. 2009; 49(1): 114–120; see here), and inhibit the proliferation of cultured tumor cells over-expressing the EGFRvIll mutation (EC50 ~75μΜ), with selectivity over other cells. D-propanolol, which is devoid of beta-blocker activity, can be used at much higher concentrations (required for cancer therapy) without precipitating prohibitive cardiac side effects. The old tricyclic antidepressant, desipramineis also a PAP inhibitor (EC50 ~20 μΜ). The drugs work by inducing endocytosis, removing 75-80% of the EGF receptor from the cell surface. D-propranolol (60 μΜ) alone or combined with desipramine (2 μΜ) administered to the cell culture for 60 min twice daily inhibited the proliferation and viability of U87-EGFRvIll cells. Chlorpromazine– also a PAP inhibitor – might be another candidate for this treatment strategy.
A Mixed Anti-Influenza Bag from INSERM
France’s Institut National de la Santé et de la Recherche Médicale is highly reputed, but not particularly well known for drug repurposing. But now WO/2012/136851(INSERM [FR]; Oct. 11, 2012) announces compositions for inhibiting influenza virus replication which are selected from gemcitabine(Lilly’s Gemzar®), obatoclaxmesylate, docetaxel, HA-14, the CDK inhibitors alsterpaulloneand indirubin 3′-monoxime, GSK3βinhibitor VIII, GSK3βinhibitor XV, reduced L glutathione, fluocinolone acetonide, the platelet aggregation inhibitor tirofiban, the antiproliferative drugs topotecan, clofarabine, vinblastine, and the vitamin K-like naphthoquinone menadione. All these inhibit viral replication in Madin-Darby canine kidney (MDCK) or A549 human lung epithelial cells, as determined by neuraminidase activity assays. The suppression effects range from impressive to dramatic for most drugs with all relevant viral strains (H1N1, H3N2, H5N1) in both cell lines, only vinblastine and menadione appear to be ineffective against H5N1 in MDCK cells.
TGF-beta Inhibitors For Schizophrenia
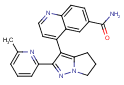 |
| LY-2157299 |
WO/2012/138945(Aestus Therapeutics [US]; Oct. 11, 2012) claims pyrazole TGF-β inhibitors from Eli Lilly & Co., including 4-[2-(6-methyl-pyridin-2-yl)-5,6-dihydro-4H-pyrrolo[1,2-b]pyrazol-3-yl]-quinoline-6-carboxylic acid amide (LY-2157299) which is currently investigated in Phase I/II for pancreatic cancer and glioma, for the treatment of schizophrenia. LY-2157299 reversed the PCP-induced deficit in prepulse inhibition (i.e., the startling reflex) at 20 mg/kg i.p. but not at 100 mg/kg. – The TGFβ pathway is most likely hyperactive in schizophrenia (Pharmacol Ther. 2009; 121(1):115-2 PubMed), which could explain these findings.



