Here is what we have for you to keep imagination going as sweltering heat sitson much of the northern hemisphere: drugs developed more than 50 years ago in France and Japan are now claimed for attention deficit disorder and urination problems, respectively; claims concerning rapamycin for schizophreniacome from China; a meloxicam-releasing contraception device has been developed in Chile; and work on mifepristone from the United States goes head-to-head with data from India.
There are very few, if any, APIs with only one activity and/or only one conceivable therapeutic use. Actually, many might have uses in therapy areas far removed from those covered by their initial development goals. H.M. Pharma Consultancy can assist you with identifying, developing and patenting such new uses — see the respective section of our website.
New For ADHD: Phace… what!?
If the name phacetoperanedoes not ring a bell, don’t worry: it was patented by Rhone-Poulenc Rorer with a February 1959 priority date (US 2,928,835) and was subsequently
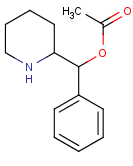 |
| Phacetoperane |
developed as an antidepressant and anorectic drug.
This isphenyl(2-piperidinyl)methyl acetate,the reverse ester of methylphenidate, which apparently shares stimulant properties with the drug marketed as Ritalin forattention deficit-hyperactivity disorder. So it might nothave been a quantum leap of inventiveness for the Paris public hospital system to claim phacetoperanefor ADHD inWO/2013/079873 (Hôpitaux de Paris [FR]; June 6, 2013)but credit goes to them for reviving this ancient agent. More to their credit, it is the dextrorotatory (S,S)-enantiomer (not the levoform that had been marketed as Lidepran) that is preferred for the purpose of the invention. In the juvenile male Wistar rat T-maze paradigm, 1 mg/kg i.p. reduced impulsivity more effectively than 3 mg/kg methylphenidate.
Lipophilic Thiamine Derivatives For Dysuria
Prosultiamine(thiamine propyl disulfide, TPD) and fursultiamine(thiamine tetrahydrofurfuryl disulfide, TTFD) are lipophilic disulfide analogs of vitamin B1, developed in Japan during the 1950s to improve treatment of Beriberi. Fursultiamine had been investigated for a repurposed application, autism spectrum disorders, before (Neuro Endocrinol Lett. 2002; 23(4):303-8, here; Neurotoxicol Teratol.2012; ;34(2):242-52, here), but WO/2013/084532 (Nagasaki University [JP]; June 13, 2013)suggests yet an entirely different range of applications – urinary disorders – for both thiamine derivatives. A 12-week treatment course significantly improved urinaryretention volume, urinationfrequency, and subjective quality of life parameters in patients with benign prostate hyperplasia and non-neurogenic overactive bladder. Not all treatment effects seem to have achieved statistical significance, but there are interesting trends here. A quick review of the peer review literature does not suggest that urologicaluses have been suggested before.
Rapamycin For Schizophrenia
In WO/2013/091334 (Wuhan University [CN]; June 27, 2013)themacrolideimmunosuppressant rapamycin (sirolimus) is claimed for schizophrenia, on the basis of corrective activity against irritability and agitation of mice caused by electrical footshocks or treatment with the NMDA antagonist, MK-801. The effective doses of rapamycin (up to 5mg/kg) did not affect behavior in animals not given such pretreatment. Is this potentially patentable, given the literature reports concerning the involvement of the mTOR (mammalian target of rapamycin) pathway in schizophrenia? At first sight it would appear so because the document’s December 2011 national priority date predates the peer review papers.A hypothesis that rapamycin may have mood stabilizing properties was disclosed in January 2012 (Med Hypotheses2012; 78(1):86-7, here). A paper reporting that recruitment of mTOR by prefrontal 5-HT6 serotonin receptors contributes to the perturbed cognition in schizophrenia (EMBO Mol Med.2012; 4(10):1043-56, here) was also published too late to interfere with novelty claims. Immunosuppression would of course be a problem during extended treatment, but then rapamycin can only be regarded as a lead structure in this context.
A Meloxicam Presentation Tuned To Contraception
Twelve years ago it was reported that 10 mg/kg of meloxicam completely inhibited ovulation in rabbits when given 5-8 hours postcoitus (Contraception2001; 63(6):329-33, here). In cynomolgus monkeys a 5-day course of oral meloxicam administered around the time of ovulation reduced the rate of oocyte release without alteration of reproductive hormones or menstrual cycle length (Hum Reprod.2010; 25(2):360-7, here), and in a pilot study conducted in women receiving levonorgestrel foremergency contraception the overall proportion of cycles with no follicular rupture or ovulatory dysfunction increased significantly by the addition of meloxicam (15 mg p.o.) (Hum Reprod. 2007; 22(2):434-9, here). WO/2013/098591 (Laboratorios Andrómaco [CL]; July 4, 2013)would appear to contain the first claims for a vaginal drug delivery presentation that is designed for constant use as a routine contraceptive. In a pilot study six healthy women received vaginal rings containing 1.75 g or 2.25 g of meloxicam, or 1.75 g of meloxicam plus 15% polyvinylpyrrolidone K-30as a release modifier.The rings containing PVP achieved higher levels of the drug in local endometrial tissue without affecting plasma concentrations of meloxicam, which remained minimal.No fertility-related data are presented.
An Abortion Drug For Heart Disease
Mifepristone(RU-486), a progesterone and glucocorticoid receptor antagonist, is the prototypical drug for inducing first-trimester abortion. In the abstract of WO/2013/103687 (Rhode Island Hospital [US]; July 11, 2013)the inventors claim “a significant breakthrough in the treatment or prevention of chronic heart failure (…) based upon the discovery that a mifepristone molecule increases the cellular level of SERCA2a protein.” – However, apaper by a group working at the Indian Institute of Chemical Biology (J Endocrinol. 2011; 209(1):105-14, here) might have preempted this byreportingthe expression of SERCA2a (the sarcoendoplasmic reticulum calcium transport ATPase 2a) to be significantly reduced in left ventricular tissues of rats treated with the glucocortocoid dexamethasone, showingthat impaired cardiac calcium kinetics could be restored by mifepristone.As the inventors note in the application, low SERCA2a activity correlates with low ejection fraction (a.k.a. systolic heart failure). It will be interesting to see if the examination report picks up on this (no international search report has been published yet).

