Today we highlight four PCT documents published between January 12 and February 2, 2012: cancer drug candidates for neglected tropical diseases; ifetroban, a THX2 receptor blocker, for hepatic encephalopathy; the oral antidiabetic, glibeclamide for peripheral and CNS ischemia and injury; and losartan for psoriasis.
Forward The Patentome!
mTOR/PI3K Inhibitors for Trypanosomatid Diseases
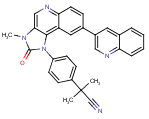 |
| NVP-BEZ-235 |
The WHO estimates that every year over 22 million people are infected with Trypanosoma brucei, T. cruzi, and Leishmaniasp. For African trypanosomiasis (sleeping sickness) there are four drugs available, most of them old and all with severe side effects: suramin (used since the 1920s) and pentamidine, introduced in the 1940s; melarsoprol (one of the very few arsenic-containing drugs still in use), and eflornithine, an irreversible ornithine decarboxylase inhibitor. For visceral leishmaniasis and Chagas disease the situation is comparable. – And now there might be a common alternative for all of these: WO/2012/006619(Northeastern Univ. [US]; Jan. 12, 2012)demonstrates that T. bruceiand L. majorpossess enzymes from the mammalian targets of rapamycin (mTOR) class that are essential for their cell growth and virulence. In addition, trypanosomatid genomes encode at least 12 proteins belonging to the phosphoinositide-3-kinase (PI3K) protein superfamily, some of which are unique to parasites. Eli Lilly’s LY294002(a morpholine derivative of quercetin), Wyeth’s WYE-354, and most of all Novartis’ imidazo[4,5-c]quinoline NVP-BEZ-235(see structure; first claimed in WO/2006/122806and now in Phase II for various solid tumors) are dual mTOR/PI3K inhibitors that were found to be highly effective. – This patent document has a July 2010 priority date, and a peer-review companion paper published in PLoS Neglected Tropical Diseasesin August 2011 (PDF).
Ifetroban for Hepatic Encephalopathy
Ifetroban(BMS 180291-02) is an injectable thromboxane A2 receptor antagonist created by Bristol-Myers Squibb which migrated to Vanderbilt University and then to Cumberland Pharmaceuticals, Inc. which in 2011 started clinical studies for hepatic encephalopathy as a complication of the hepatorenal syndrome (press release). The new application seems plausible (but also non-trivial) considering that the literature on this drug almost exclusively concerns endothelial dysfunction in the context of general cardiovascular conditions. The rationale is that high levels of liver-derived isoprostanes mediate microvascular constriction and permeability via thromboxane receptor activation, and that an antagonist will normalize cerebral blood flow. A Phase II study (NCT01436500) is currently recruiting patients, and is scheduled for completion in October 2015. WO/2012/009545(Cumberland Emerging Technologies [US], Jan.19, 2012)is the IP document covering this new indication.
Glibenclamide Infusion for Ischemic Attacks and CNS Injury
Glyburide (glibenclamideoutside the U.S. and Canada) is an old sulfonylurea-class oral antidiabetic drug. In WO/2012/012347(Remedy Pharmaceuticals [US]; January 26, 2012)the formulations are intravenous infusions (development code RP-1127) for emergency use in stroke, traumatic brain or spinal cord injury, myocardial infarction, hemorrhagic shock, and acute ventricular arrhythmia. Phase II studies are recruiting patients for the two first-mentioned indications (NCT01454154for TBI and GAMES-PILOTfor stroke). An earlier Phase I study has been concluded, from which the patent document discloses pharmacokinetics data. The staged 0.4 mg/day dose regimen according to the invention builds glibenclamide plasma concentrations of 4-5 ng/ml within 15 hours. This dose had a very minor effect on blood glucose levels; it was more pronounced with the 3.0 mg/day dose but still without hypoglycemia, which would be damaging in ischemic conditions. The inventors believe that glibenclamide levels of approx. 25 ng/ml should be targeted. – It is well known that the sulfonylurea receptor 1 (SUR1) plays a key role in various forms of CNS injury (see here). Specifically, glibenclamide has been shown to reduce hippocampal injury and to preserve spatial learning in a model of traumatic brain injury (see here). The potential effects of anti-diabetic medications on myocardial ischemia-reperfusion injury have recently been discussed (see here), and its has been demonstrated that exenatide (also a K(ATP) channel opener) prevents endothelial dysfunction in a human model of peripheral ischemia-reperfusion injury (see here).
Losartan for Psoriasis
WO/2012/013990(indiv. inventors [GR]; Feb. 2, 2012) is one of those patent applications that make you scratch your head. Titled “Medication for curing the disease of psoriasis,” it has exactly three pages (two if you don’t count the front page), gives no data and cites no literature. It simply states that losartan potassium (100 mg/day p.o.) was given to 21 hypertensive psoriasis patients, and that 20 “noted immediate regression, almost after the first week of the medication treatment. Whithin a period of 90 days, all the symptoms of the disease of psoriasis were disappeared.” (sic) – That would be notable indeed because what little linkage can be found in the literature points exactly to the opposite: angiotensin receptor II antagonists can induce psoriasis (see hereand here). Any further details from the Greek inventors (who have not published in PubMed-listed journals) would be much appreciated. Indeed, learning about finding related to these claims would be a good application of life science crowdsourcing. If you have something to add, please use the blog comment section or our contact (below).
H.M. Pharma Consultancy is a leader in knowledge extraction from drug repurposing patent documents, and gives strategic advice to developers looking for repurposing opportunities. Find out more at www.hmpharmacon.comor contact us at office@hmpharmacon.com.

