Ah, what a month January has been! The power of networking and collaboration is making itself felt. Our business development path until midyear has been mapped, as far as such planning can go. No guarantees of course – there are no such things if you are working in the truly independent line. To paraphrase Clausewitz’ pun on battles, no business plan survives contact with reality as long as you are treading unknown ground.
You will have noticed that this blog has been given a new design — simpler, fewer gadgets, easier to read, and with all the options to switch between views accessible from the menu. The color scheme is now better aligned with that of our Twitter site – and of course with that of our website.
But that was a matter of minutes. Other things have been making more demands on our time and creativity. Transitioning our general patent reviews to the soon-to-be-launched journal, Pharmaceutical Patent Analyst has required some tweaking of our formatting to meet the standards of the Future Science Group. But thats good, such things force us to reconsider well-trodden paths. In addition, refocusing the more informal patent highlights on this blog exclusively on IP documents that touch on drug repurposing has tickled our creative thinking in more than one way.
 |
| Off-Target Drug Repurposing |
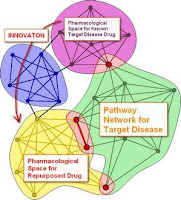
If you are looking for openings in this field each such published patent application speaks of a missed opportunity: “Hey, I might have had this idea myself!” – Never mind that; the intersection of unmet need space in medicine and the unexploited pharmacological potential of known compounds will not be exhausted for many years to come. As you might remember from some of my earlier ramblings here, we believe intelligent prior art search in our own focused databases and exhaustive text analysis – in conjunction with know-how in drug development – to provide as a distinctive edge in drawing up concepts for drug repurposing. The figure illustrates one potential embodiment of this strategy.
We have formalized several concrete project proposals for redeveloping drugs or drug candidates – some discontinued, some not -, and these have already attracted enough attention to be tested by interested parties. If, when and how we can move forward with any of these depends [A] on the outcome of the experiments (to be awaited), [B] on the tenacity of our development partners (beyond doubt of course), and [C] on the degree of external funding that can be racked up this year or in early 2013 — provided that the science can be made to work. Expressions of interest to that effect are of course invited.

