This third issue of our monthly Life Science Patent Highlights. As always, the most recent documents might become available at the European Patent Office only a few days after this analysis goes online.
PLEASE CITE AS: Mucke HAM. Patent Highlights for January. Published online in the H.M. Pharma Consultancy Blog (http://hmpharmacon.blogspot.com) on February 26, 2011. Contact us at office@hmpharmacon.com .
An Insight Into Bitterness
With a limited repertoire of about 30 taste receptor genes, humans can detect thousands of different bitter-tasting compounds. This promiscuity results from a survival-promoting evolutionary mechanism that allows humans to recognize toxic compounds with chemical structures as widely diverse as those of e.g. cycloheximide, various alkaloids, and cyanide — all of which have an highly aversive bitter taste. For the pharmaceutical industry this has turned into a legacy; many drugs have a very bitter taste that requires addition of masking agents in high concentrations if they are to be fomulated as oral solutions or buccal presentations. It would be much more reasonable to use specific receptor blockers. WO 2011/012298 (German Institute for Nutrition Research [DE]; Feb. 3, 2011) focuses on a particular receptor known as TAS2R49, and describes methods for identifying agonists that are structurally related to the anti-asthma agent cromolyn or the antiemetic muscarinic antagonist diphenidol by their interactions with expressed subunit polypeptides. These compounds are then modified to become antagonists. Obviously such compounds could also make bitter-tasting foodstuff more palatable — but unless they are already known and “generally recognized as safe” (GRAS), they could hardly be legally used for such purposes. The institute has two related international patent applications, WO 2010/099983 and WO 2008/128730.
A Lotion To Detect Emerging Pressure Sores
Decubitus ulcers, also known as pressure sores or bed sores , are the bane of bed-ridden patients and persons restricted to wheelchairs. Once developed, decubitus lesions can take months to heal. Dark-skinned people are at particular risk because the earliest stages of decubitus are not visible even to nurses trained in skin assessment.WO 2011/014552(Individual inventor [US]; Feb. 3, 2011) offers an ingenious solution: a thermochromic lotion (based on liquid crystals, microparticles or leuco dyes) is applied to the skin areas that make contact with surfaces, and the result is read by visual comparison with a color chart. This allows decubitus to be diagnosed in Stage I (blanchable erythema from reactive hyperemia), when its progression is easily preventable by massage, skin care and a turning schedule for the patient. There might be additional uses in the diagnosis of hypothermia for detection and grading of frostbite, in the treatment of chemical burn victims where the total area of exposure may be unknown or undetectable to the eye, and in the diagnosis of radiation injuries to skin.
Dashurin, A New Biomarker For Renal Injury
Although serial measurement of serum creatinine is an accepted method of detecting and diagnosing acute kidney injury, this marker can take two days or more for serum creatinine to rise to values that are considered diagnostic for injury and prognostic for the development of acute renal failure. Urine biomarkers which are closer to real-time processes would be needed. But what is dashurin? WO 2011/017614 (University of Vienna [AT]; Feb. 10, 2011) is the only document in the entire PCT database that mentions this name. PubMed returns exactly one paper, Biochim Biophys Acta. 2010; 1800(4): 430-8 (see here ) which names two of the inventors as authors. The patent document gives sequence information for an open reading frame of 945 bp coding for 314 amino acids of a protein (the first 28 aa are a signal protein) which is expressed in glomeruli. Antibodies and immonoassays for dashurin are presented.
A Protein To Activate Gamma Secretase
WO 2011/016861 (Intra-Cellular Therapies, Inc. [US]; Feb. 10, 2011 ), the companion patent application to a Nature paper from Rockefeller University (see here ) which was published last September, deals with a novel γ-secretase activating protein (gSAP) that selectively modulates Aβ production. This happens through a mechanism involving its interactions with both γ-secretase and its substrate, amyloid precursor protein C-terminal fragment (APP- CTF). gSAP does not interact with Notch, which makes it a potentially attractive target for the treatment of Alzheimer’s disease. Interference with this interaction between gSAP and APP- CTF appears to be the mechanism by which the cancer drug imatinib(Novartis’ Gleevec) achieves its amyloid-beta-lowering effect. N-(6-methyl-5- (4-phenylpyrimidin-2-ylamino)pyridin-3-yl)-4-((1 -methylpiperidin-4-yl)methyl)benzamide significantly lowers Aβ at concentrations of <500 nM. Also claimed is a method of identifying persons at risk of developing Alzheimer’s disease based on single nucleotide polymorphisms of gSAP. Intra-Cellular Therapies banks on the work of 85-year old Paul Greengard, Rockefeller University’s Vincent Astor Professor at the Laboratory of Molecular and Cellular Neuroscience and the winner of the 2000 Nobel Prize for Medcine, who heads the company’s scientific advisory board.
Nucleic Acid Therapy For Ebola Infection
Filoviridae such as Ebola and Marburg viruses cause only a few hundred reported deaths worldwide each year and as such they are hardly among the most pressing global infectious disease agents. However, especially Ebola has characteristics that could make it suitable as a biological weapon — and once biodefense is involved there is enough research funding to make development of countermeasures a viable proposition for the pharmceutical industry. A case in point is WO 2011/020023 (Alnylam Pharmaceuticals [US]; Feb. 17, 2011) which describes lipid formulations of double-strand RNAs that are 19-24 nucleotides long, have one strand that is complementary to an Ebola virus gene (e.g., the L gene) and inhibit its expression of by at least 40%. The dsRNA can be administered at doses of 0.03-3.0 mg/kg. Data from mice and guinea pig models with formulations based on N,N-dimethyl-2,3- dioleyloxy)propylamine (DODMA) and liposomes are presented. This application seems to stem from the Alnylam Biodefense Initiative for which the company won two NIH contracts for a total of more than $63 million in 2006 and 2007; this proceeds under the umbrella of the U.S. Defense Threat Reduction Agency’s Transformational Medical Technologies Initiative ( TMTI). For RNAi approaches to the Ebola L gene see J Infect Dis 2006; 193(12): 1650-7, here.
CNS Drug Delivery Through The Ear
The U.S. Food and Drug Administration recognizes more than 100 routes of drug delivery, and the first on the alphabetical list is the auricular route. However, WO 2011/019954 (Individual inventor [US]; Feb. 17, 2011) is not directed to otic infections or inner ear problems such as tinnitus – it aims at the delivery of brain-targeted drugs, formulated as solid lipid nanoparticles, to the cerebrospinal fluid via the perilymphatic compartment. This should be possible because the cochlear aqueduct connects the inner ear and the subarachnoidal space. Wearable thermoelectric dispensing systems with electronic control are claimed which look very much like a typical behind-the-ear hearing aid. A conduit is implanted in the tympanic membrane and configured to convey fluid from the ear canal to the round window membrane. The application focus is on the treatment of intractable cancer pain with opioids; however, eating disorders and obesity are also exemplified. For a related 2007 paper in Acta Pharm Sinica , see here. Given the absence of supporting biological data in the application it is difficult to judge if this route could be as effective as intracisternal delivery. The inventor has no papers listed in PubMed but is namend on 14 international patent applications, most of which deal with aerosolizing devices and are assigned to Aerogen, Inc., a company he had founded in 1991.
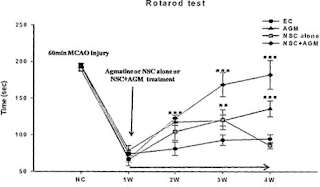
Agmatine Promotes Stem Cell Efficacy and Survival
Agmatine (1-amino-4-guanidinobutane) has many roles: it is an is an endogenous neuromodulator with affinity to alpha(2)-adrenergic, imidazoline and NMDA receptors, and it inhibits nitric oxide synthases. WO 2011/019124 (Yonsei University [KR]; Feb. 17, 2011) now teaches that agmatine not only promotes the differentiation and colonization of transplanted neuronal stem cells, but also their post-transplant functional survival (see figure). Considering that the protection of retinal ganglion cells by agmatine had been claimed (also be Korean inventors) in WO 2008/123684, this does not come entirely as a surprise. However, no such action on stem cell survival seems to have been reported so far. For more remotely related claims for agmatine see WO 2001/095897 (seizures) and WO/1999/008669 (neuropathic pain)
Insect Models for Pharmacokinetics and Blood-Brain Barrier Penetration Testing
WO 2011/018446 and WO 2011/018450 (Entomopharm [DK]; Feb. 17, 2011) outline the interesting business concept of this Copenhagen company, which was established in 2009: to use insect systems for drug discovery screening. For PK studies, a locust or cockroaches are administered test compounds and subsequently hemolymph is obtained for analysis by puncturing the ventral membrane between the head and the thorax.Locusta migratoriahad been used to develop and validate the model. An ex vivo approach was chosen for BBB penetration testing: the dorsal part of the insect head is dissected to expose the brain, eyes, antennas, and nerve associations. The brain is exposed to the test compound solution while still in its cuticle shell, and then homogenized for assaying. A large number of examples show that typical CNS active compounds such as mianserin, caffeine, trazodone, buspirone, and propranolol permeate the grasshopper BBB to a much larger extent than peripheral acting drugs such as colchicine and atenolol. This type of testing could save large numbers of rodents in the drug discovery stage, and would be the ideal second stage to cell culture-based high-content screening.
Antidotes for Botulism Toxins
WO 2011/022721 (Microbiotix [US]; Feb. 24, 2011) is another application related to bioterrorism countermeasures. In the case of Botulinum neurotoxins (two-chain polypeptides with a heavy chain joined to a zinc-dependent endopeptidase light chain which impairs neuronal exocytosis through proteolysis of essential components of neurotransmission) , their development essentially focuses on preventive vaccines and passive immunotherapy with “despeciated” F(ab)2 antibody tragments. Here comes what, especially could be a very viable alternative to these biotech products which are less than ideal under conditions of public mass emergency or combat: simple organic compounds that inhibit the toxin’s proteaolytic activity, and work particularly well for serotype A. Data are presented for 5-chloro-7-((pyridin-2-ylamino)(thiophen-3-yl)methyl)quinolin-8-ol (MSL-151862), 2-(lH-benzo[d]imidazol-2-yl)-3-(3-iodo-4-methoxyphenyl)acrylonitrile (MSL-145815), and (E)-3-( [2,2′-bithiophen] -5-yl)-2-(1H-benzo[d] imidazol-2-yl)acrylonitrile (MBX 1553). IC50 values are mainly in the 10.75 µM range.